Following a healthy lifestyle has been a growing trend for many years, with more individuals seeing health as a new state of mind and body. The recent epidemic has exacerbated the interest in proactively checking one’s health status. The medical devices market volume is expected to reach US$435 million in 2022 and experience an annual growth rate of 5.32% by 2026.
As health check-ups become more frequent, there is also a growing need for medical devices that are small, portable, and yet reliable. Reliability can not only impact the lifespan of a medical device. This factor can also influence a patient’s and operator’s safety or the accuracy of the data collected at a given time.
For this reason, engineers need to consider a plethora of factors to guarantee sufficient levels of safety and high reliability in their electric circuit projects for healthcare and medical device applications.
The Classification of Medical Equipment
Medical electrical equipment is essential for the diagnosis, treatment, and monitoring of a patient’s health status. Most medical equipment can be classified into three main categories: diagnostic, treatment, and auxiliary equipment. Each macro category can in turn be divided into a series of subsystems based on the exact application of each medical device.
According to the MDCG 2021-24 recently released by the Medical Device Coordination Group (MDCG), medical equipment can be further differentiated into more categories based on its duration of use and level of invasiveness. In terms of duration of use, medical equipment can be divided into transient use (for continuous use for less than 60 minutes), short-term use (for between 60 minutes and 30 days), and long-term use (for more than 30 days). The level of invasiveness and duration of use should be among the many factors to consider for designing reliable medical equipment circuitry.
The level of risk for users is another crucial factor that is often considered to classify medical devices and determine the exact safety requirements. For example, according to the FDA, medical devices can be divided into three classes according to the risks associated with each device. The higher numbered class, the greater the regulatory control and the stricter the requirements:
- Class I medical devices with a low to moderate risk to the user (approximately 47% of medical devices regulated by the FDA)
- Class II medical devices with a moderate to high risk for the user (approximately 43% of medical devices)
- Class III medical devices with a high risk to the user (approximately 10% of medical devices)
Besides regional regulations for the classification of medical electrical equipment, several international standards also guide electrical engineers and device manufacturers on critical matters like product safety. Among the many international standards, the IEC 60601-1 are the most prevalent standards to consider for ensuring maximum reliability and safety of medical electrical equipment. Below we will review more in detail this and other related standards.
The Standards to Guarantee Higher Safety in Medical Power Supplies
The IEC 60601-1 obligates medical electrical equipment manufacturers and designers to ensure that they are completely safe for the patient and the user. Specifically, this standard regulates the requirements for connectors and other components that handle the transmission of electrical power to provide protection against electric shock in point-of-care scenarios where medical come in contact – or could potentially come in contact – with a patient or operator.
Some examples of equipment that should comply with the IEC 60601-1 standard include heart and blood pressure monitors, surgical lasers, and diagnostic ultrasound equipment. This specification covers not only the power supply unit itself but also the equipment it is powering. In other words, the qualification applies to the complete system in a medical and healthcare device, and thus it should also apply to external power supplies and wall-mounded power supply adapters.
The IEC 60601-1 standard can also be associated with other similar standards (including the IEC 60601-1-1, the IEC60601-1-2, IEC60601-1-3, IEC60601-1-4) to specify further the exact requirements for the different categories and sub-categories of medical electrical equipment. These harmonized standards are recognized worldwide, while also showing several localized deviations in key markets. 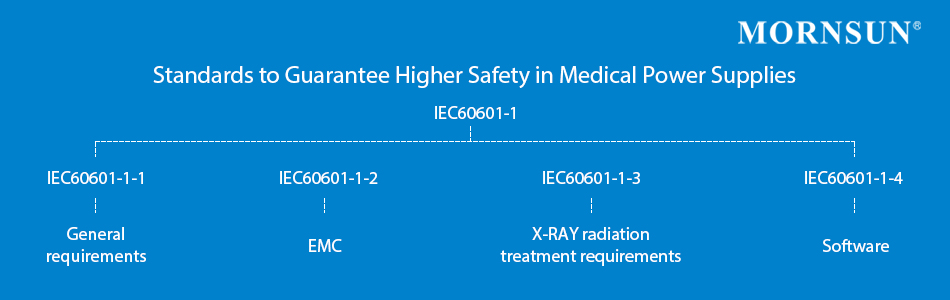
Within the IEC 60601-1 suite of standards, there is a specific distinction to consider for the means of protection (MOP) required for each type of medical electrical equipment. This protection includes insulation, clearance, creepage distances, impendances, and protective earth connections. The two primary safety classifications include:
- MOPP (Means of Patient Protection)for medical electrical equipment with direct patient contact that must fulfill the highest safety requirements;
- MOOP (Means of Operator Protection)for medical electrical equipment without direct patient contact that must nevertheless fulfill high safety requirements;
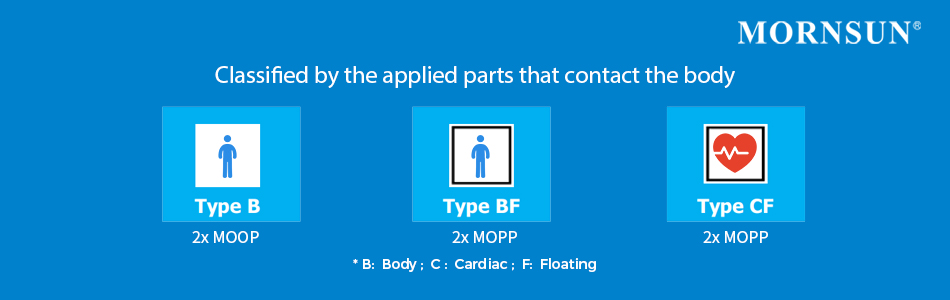
From this classification, it is possible then to distinguish between parts that can come in contact with the Operator and parts that can come in contact with the Patient, as well as “Applied Parts”. “Applied Parts” refer to those parts that come in contact with the patient in order for the medical equipment to perform its intended function. The Applied Parts classifications can be divided into several types:
- Type B (Body) –This type of classification is the least stringent as it is often associated with applied parts that are generally not conductive and may have a reference to the ground;
- Type BF (Body Floating) – This type refers to classified parts that are electrically connected to the patient, not in direct contact with the patient’s heart, and are often floating or separated from the ground;
- Type CF (Cardiac Floating) – This is the most rigorous classification, which refers to applied parts that are suitable for direct cardiac and intravenous connection.
The IEC 60601-1 standard provides medical electrical equipment manufacturers with reference to categorize the likelihood of a patient coming into contact with the product and the consequential risks.
Medical device designers can employ these standards to decide whether to use patient protection (MOPP) or operator protection (MOOP) and the insulation levels required. In both cases, the insulation between primary to secondary should meet at least 2 MOP, which can also differ in based on the applied parts to which it should be applied. Type-B equipment requires a 2x MOOP, while type-BF and type-CF medical equipment necessitates an insulation resistance level of 2x MOPP.
Engineers should consider all of the above-mentioned factors, the applicable medical regulations and standards, as well as the specific application of their medical device circuit design projects. There might be significant differences also in the safety requirements of medical devices intended for either medical or commercial applications.
When selecting power supplies for medical electrical equipment, engineers should also consider low leakage current levels, sufficient safety distance (both in terms of clearance and creepage distance), high temperature protection, and an isolation voltage higher or equal to 4000V.
MORNSUN’s Power Supply Solutions
Engineers looking to design an electrical circuit for medical and healthcare equipment should look for specialist power supplies with high-caliber design and performance. The recommended solutions differ according to the exact medical application, with a special focus on patient-connected systems.
The general advice is to select standard, approved, suitably rated, and medically approved power supplies to ensure the highest safety possible for medical equipment users. Power supplies that are IEC-60601-1 certified and with proven EMC performance should be one of the top choices for electrical engineers. Relying on qualified and experienced medical power supply manufacturers like MORNSUN is also crucial to designing the best performing and lowest-risk circuit design for a medical and healthcare device.
MORNSUN has over 23 years of experience in delivering reliable and safe power supply solutions for a wide range of applications, including for medical and healthcare devices. The MORNSUN power supply solutions space from AC/DC converters to DC/DC converters and other electrical components.
Contact us to ask us any questions or request a sample of our power supplies for medical and healthcare devices.
or becoming even smaller. This trend leads the way for railway power supplies with higher power den
For more information, please visit www.mornsun-power.com










